May 2020: SanoStem has reached an agreement with Stemedica to license its technology to build a cGMP manufacturing plant in India. On completion of a successful clinical trial using itMSC for COVID-19-related lung disease and ARDS, SanoStem will request permission to build a manufacturing plant in India.
Stemedica’s itMSCs and itNSCs are manufactured in a cGMP compliant facility licensed by the State of California Department of Public Health Food and Drug Branch (FDB).
Stemedica manufacturing process itMSCs and itNSCs:
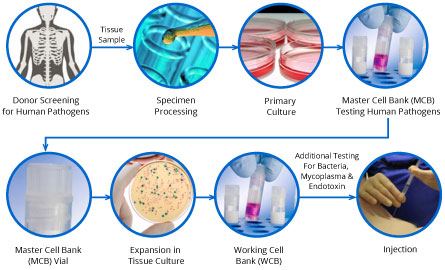
Stemedica’s Technology Platform includes core enabling competencies, proprietary methodologies, expertise and intellectual property related to its manufacturing processes and procedures conducted at its own facilities.
The Technology Platform delivers quality, safety and reliability with:
- Manufacturing in its own cGMP compliant facilities
- Licensing by U.S. FDA and Swissmedic for pre-clinical and clinical use in approved trials
- Safety in full compliance with U.S. FDA, FTB, Swissmedic and European Union requirements
- Multiple stem cell lines
- Product Development: proprietary protocols and standard-operating procedures for isolation, expansion, preservation and master banking
- Hypoxic-controlled environments
- Specialized media
- Clone-enrichment methodology
- Scalability at commercial levels
- Documented traceability
- Documented reproducibility
- Bio-safety testing at certified laboratories
- Preservation and master banking
- Global shipping and receiving methodologies
Stemedica’s manufacturing process offers stem cells with these benefits:
- Safe and powerful expansion process
- No contamination
- Consistency across multiple lots
- High potency for studied indications
- Unmatched capacity

